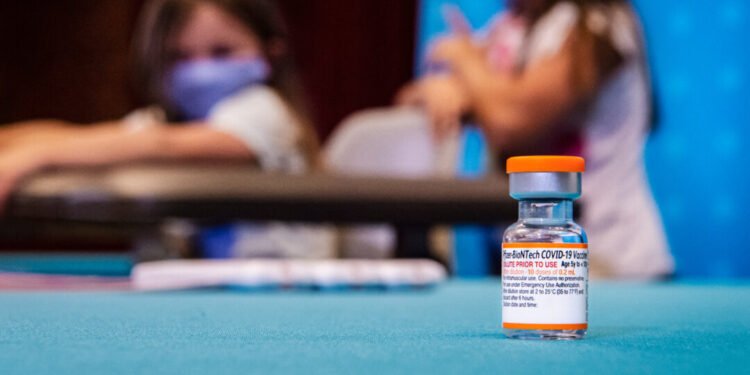
In a groundbreaking acknowledgment that marks a significant shift in federal health policy discourse, the Food and Drug Administration has officially determined that COVID-19 vaccines were responsible for at least 10 deaths among children, representing the first time U.S. health officials have formally established a causal link between the vaccines and pediatric fatalities.
This unprecedented admission by federal regulators comes after years of maintaining that serious adverse events in children following COVID-19 vaccination were extremely rare and that the benefits of vaccination far outweighed potential risks for the pediatric population.
The FDA’s conclusion represents a pivotal moment in the ongoing evaluation of COVID-19 vaccine safety, particularly as it relates to the youngest Americans who received the shots. The agency’s findings are expected to intensify debates surrounding vaccine recommendations for children and may influence future policy decisions regarding pediatric immunization programs.
Since the rollout of COVID-19 vaccines for children began in late 2021, health authorities have consistently emphasized the safety profile of the vaccines while acknowledging that rare adverse events could occur. The Pfizer-BioNTech vaccine was initially authorized for children aged 5-11 in October 2021, followed by authorization for younger age groups in subsequent months.
The FDA’s determination comes as part of ongoing safety monitoring efforts that include analysis of reports submitted to the Vaccine Adverse Event Reporting System (VAERS) and other surveillance databases. These systems are designed to detect potential safety signals and investigate reported adverse events following vaccination.
Medical experts have long emphasized that establishing causality between vaccines and adverse events requires careful investigation and analysis of multiple factors, including medical history, timing of events, and alternative explanations for reported outcomes.
The acknowledgment of vaccine-related deaths in children is likely to fuel discussions about informed consent, risk-benefit calculations for different age groups, and the transparency of vaccine safety data. Parents, healthcare providers, and policymakers will be closely watching for additional details about the specific circumstances surrounding these cases.
This development occurs against the backdrop of broader conversations about COVID-19 vaccine policies and safety surveillance, with various stakeholders calling for enhanced transparency in reporting and investigating adverse events across all age groups.
The FDA’s findings are expected to prompt further scrutiny of pediatric vaccination programs and may influence recommendations from the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices, which provides guidance on vaccine use in the United States.
As this story continues to develop, health officials, medical professionals, and families across the nation will be seeking additional information about the specific details of these cases and what implications they may have for future vaccination recommendations and safety protocols.